Frustrated with "rust-proof" blades showing rust spots? This unexpected corrosion can halt production and increase costs. Let's explore the simple reasons why and how to prevent it.
Stainless steel rusts when its protective chromium oxide layer1 is damaged, allowing iron to react with moisture and oxygen. To prevent this, always clean, dry, and lightly oil your blades after use. Avoid harsh chemicals and store them properly to maintain this protective layer.
The term "stainless" can be a bit misleading. It should really be called "stain-resistant." This is a lesson one of my customers, Glenn, learned the hard way. He is an equipment supervisor at a food processing plant in the US and was baffled when his stainless blades started showing rust spots in their humid environment. I explained to him that the problem wasn't the steel itself, but how it was being treated. At PASSION, we believe that empowering our customers with knowledge is just as important as providing them with precision blades. Let's break down exactly what's happening to your blades so you can protect your investment.
How Does The 'Protective Shield' On Stainless Steel Work, And What Breaks It?
You trust your stainless steel blades to be tough all the way through. But even the strongest armor has a weak point. Let’s look at this invisible shield.
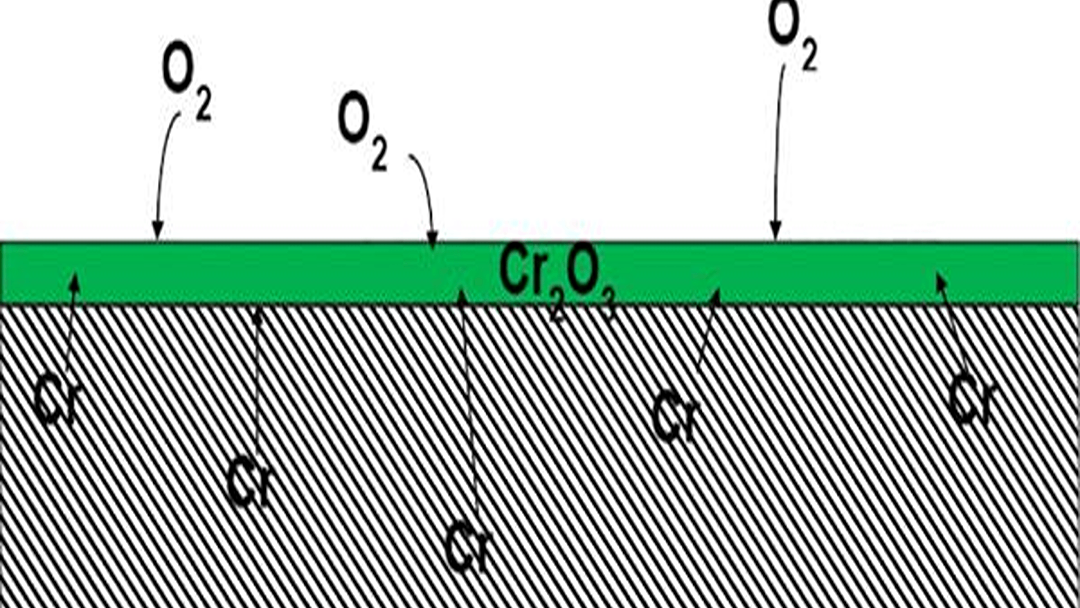
The protective shield is a thin layer of chromium oxide that forms on the surface, preventing rust. This shield can be broken by physical scratches, prolonged exposure to moisture2, and contact with corrosive substances like salt, acids, and especially chlorides3, leaving the iron underneath vulnerable to rust.
Think of your stainless steel blade not as a single material, but as a fortress. The steel itself is strong, but its defense against rust comes from a very thin, invisible layer on the outside.
What Is This "Passive Layer"?
This shield is called a "passive layer4" or "chromium oxide layer." The chromium inside the steel alloy reacts with oxygen in the air. This reaction creates a stable, non-reactive film that covers the entire surface. This film is like a perfect coat of paint that seals the iron in the steel away from air and water. As long as this layer is intact, the iron cannot rust. It even has a 'self-healing' ability; if scratched, the exposed chromium will react with oxygen again to repair the shield, but only under the right conditions.
Common Threats To The Protective Shield
Unfortunately, this shield isn't invincible. In tough industrial environments like Glenn's food processing plant, it's constantly under attack.
| Threat | How It Damages The Shield |
|---|---|
| Mechanical Damage | Scratches or scuffs from use or improper storage scrape the layer off. |
| Prolonged Moisture | Constant dampness prevents the shield from 'healing' itself properly. |
| Acids and Salts | Food residues (like citrus or brines) can chemically eat away at the layer. |
| Chlorides | Aggressive chemicals, like bleach, completely destroy the passive layer. |
What Simple Daily Habits Can Dramatically Extend The Life Of Your Stainless Steel Blades?
Worried that proper blade care is too time-consuming? Ignoring simple maintenance can lead to premature failure and costly downtime. Here are a few quick habits that make a huge difference.
Immediately after use, rinse blades with clean water to remove any residue. Dry them completely with a clean cloth. Applying a thin coat of food-grade mineral oil provides an extra barrier against moisture. Finally, store them in a dry, protected place away from corrosive elements.
After explaining the 'why' of rust to my customers, I always follow up with the 'how' of prevention. You don't need a complex or expensive maintenance routine. In fact, the most effective methods are often the simplest. Consistency is what truly matters. By building these four small habits into your daily workflow, you create a powerful defense against corrosion that will save you time and money. It's the core of the maintenance plan we developed with Glenn, which completely solved his rust problem.
Step 1: Rinse Immediately
Don't let residue sit on the blade. Whether it's food particles, adhesives, or other materials, they can hold moisture and acids against the steel. A quick rinse with clean water right after a production run removes these threats before they can do any damage.
Step 2: Dry Thoroughly
This is the most critical step. Rust is iron oxide, and you can't have oxidation without oxygen and water. Simply wiping the blade down with a dry, clean cloth removes the water from the equation. Air-drying is not enough, as it leaves the blade exposed to moisture for too long.
Step 3: Oil Lightly
For an extra layer of protection, especially in humid environments, apply a very thin film of food-grade mineral oil. This oil acts as a physical barrier, repelling any stray moisture that might land on the blade during downtime.
Step 4: Store Properly
Never toss blades into a pile or leave them on a damp surface. Store them in a dry, designated rack or sheath. This not only protects them from ambient moisture but also prevents them from getting scratched or nicked, which would damage the protective passive layer.
Are You Accidentally Destroying Your Blades With The Wrong Cleaning Products?
You’re diligent about sanitation, using powerful cleaners to keep things sterile. But what if your cleaning routine is secretly causing the rust you’re trying to prevent?
Yes, it's highly possible. Many industrial sanitizers and bleaches contain chlorides. Chlorides are extremely aggressive and quickly destroy the blade's protective chromium oxide layer. This chemical attack is one of the fastest ways to cause pitting and widespread rusting, even on high-quality stainless steel.
This is one of the most common issues I encounter, especially in the food processing industry where sanitation is a top priority. A customer will tell me they clean their blades meticulously, yet the rust keeps getting worse. Often, the culprit is the very cleaning solution they are using to help.
The #1 Enemy: Chlorides
The single worst enemy of stainless steel's protective layer is the chloride ion. And where do you find chlorides? The most common source is bleach (sodium hypochlorite), but they are also active ingredients in many sanitizing agents and some detergents. While excellent for killing germs, chlorides aggressively attack and break down the chromium oxide layer, causing tiny pits and cracks. Once the shield is breached, rust can form very quickly, and since it starts in these tiny pits, it can be hard to spot until it's a serious problem. You must never use bleach or chlorine-based cleaners on your blades. Always check the label of your cleaning agents.
The Proactive Solution: Regular Passivation
What if your blades are already showing small rust spots or have been exposed to harsh chemicals? You might not need to replace them. For situations like Glenn's, we sometimes recommend a process called passivation. This involves using a special cleaning agent (like a citric or nitric acid-based gel) to perform a deep clean. This process removes any free iron and contaminants from the surface and helps the steel form a new, thicker, and more uniform protective chromium oxide layer. It's like a factory reset for your blade's rust resistance. By combining a better blade material (higher chromium content) with a chloride-free cleaning process and a simple passivation schedule, Glenn's blades now remain as bright and clean as the day they were installed.
Conclusion
Stainless steel rusts, but it's preventable. Understand the protective layer and practice proper care—clean, dry, oil, and avoid chlorides—to ensure your blades last and perform perfectly.
Learn about the crucial role of the chromium oxide layer in preventing rust on stainless steel. ↩
Find out how moisture impacts stainless steel and ways to mitigate its effects. ↩
Explore the dangers of chlorides and their impact on the longevity of stainless steel. ↩
Discover the science behind the passive layer and its importance in rust prevention. ↩